Smart measurement technology for oncologic patient care

The Dutch Cancer Society (KWF) is organizing a thematic call ‘Smart Measurement Technology (SMT) for oncologic patient care’ 2024 to catch up with the rapidly developing health care field. Following two successful rounds, we have adjusted our ambitions and criteria for this, most likely final, smart measurement technology related call, which can be read below.
Problem
The number of patients living with (the consequences of) cancer, as well as the population identified with a high risk of developing cancer, is still increasing. Patients often undergo multiple rounds of treatment and consecutive monitoring in an hospital setting, impacting patients’ lives severely. At the same time, different types of smart measurement technologies with potential opportunities for these patient groups are being developed. Smart measurement technologies include biosensors, wearables, patches, and other home monitoring tools. Unlike data collected at a single clinic visit, smart measurement technologies can collect abundant real-time data that can be utilized for various purposes in oncologic care. Moreover, minimal invasive measurements by smart technologies can reduce visits to the hospital, which not only affect quality of life, but also have logistical and societal consequences. While clinician’s and patient‘s needs determine the unmet clinical need, clinicians usually lack the expertise of engineering technologies. On the other hand technical developers have the expertise, but often lack the knowledge to translate technologies into a clinical application in the field of oncology. In other words, there is an urgent need to facilitate development of new smart measurement technologies that can be implemented into clinical practice.
Now is the time to connect the world of technology with challenges in clinical practice keeping the patient journey at the forefront and building towards smart technology driven solutions. To achieve this, KWF opens this call to attract multidisciplinary teams to further advance or repurpose technical development, validation and implementation for oncological care. We need to further develop impact-driven projects rather than technology-driven projects.
Aim
The aim of this call is to stimulate the further development of smart measurement technology to provide (early) diagnosis and monitoring of oncological patients in a minimally invasive manner and closer to home.
Ambition
The Dutch Cancer Society (KWF) wants to give an impulse to further develop necessary smart measurement technology. KWF would like to stimulate the collaboration of an interdisciplinary team of (technological, social, and/or biomedical) researchers, patients, healthcare professionals, and potential industrial stakeholders to enable further development of smart measurement technologies in oncological care. As such, the focus of this call is the utilization of smart measurement technology to aid screening and diagnosis of high-risk population/groups, and monitoring of patients living with (the consequences of) cancer. We envision multidisciplinary teams, that fulfil the unmet clinical needs with state-of-the-art technologies. This call is to further advance technical development, validation and implementation in practice, or technology repurposing for oncology care. The proposal will be preceded by a pre-proposal stage, which allows prioritization if necessary.
Requirements for research projects
- TRL: 4-6
- Project duration: 1-4 years
- Indicative budget per proposal: €200-600k
- Research phase*: Creation of modality
- Participating parties:
- Technical researcher (demonstrated scientific background)
- (Bio)medical researcher
- Optional parties:
- Public/Private collaborations are possible, for-profit private partners can participate and this is encouraged (see additional conditions).
- (Psycho)social researchers (inclusive design)
- Participation of patients. However, description of how to meet patients' needs with the innovation is required.
- Other:
- Clear developmental and sustainability plan applicable especially for oncological care. The development plan clearly describes the next phase if the project shows potential. In the sustainability plan it is described how this idea can fit into the current care setting and can be scaled up nationally.
- Information on how this innovation will be socially and societally embedded must be provided.
- Inclusion of (early) Health Technology Assessment to establish cost effectiveness.
* See Guidelines for research phase definitions
Requirements for consortium projects
- TRL: 6-8
- Project duration: 1-6 years
- Indicative budget per proposal: €400k-1.5M
- Research phase*: Preclinical/clinical
- Participating parties:
- Technical researcher (demonstrated scientific technological background)
- (Bio)medical researcher.
- Patient participation at the forefront and included throughout the project.
- A project manager must be appointed
- Optional parties:
- Involvement of (psycho)social researchers are also highly encouraged (inclusive design).
- Public/Private collaborations are possible, for-profit private partners can participate and this is encouraged (see additional conditions).
- Other:
- Clear developmental and sustainability plan applicable especially for oncological care. The development plan clearly describes the next phase if the project shows potential. In the sustainability plan it is described how this idea can fit into the current care setting and can be scaled up nationally.
- Information on how this innovation will be socially and societally embedded must be provided.
- Inclusion of (early) Health Technology Assessment to establish cost effectiveness.
* See Guidelines for research phase definitions
Topics within scope:
- Examples of smart measurement technology; smartwatch, dipstick, patches, signature analyses, tabletop devices for measurements at home or closer to home.
- Urine, blood, saliva tests in home situation with companioning logistics of transportation etc.
- Repurposing of existing technologies for oncology.
- (Early) diagnosis of (recurrent) disease and monitoring (incl. toxicity) during and after therapy and/or patient selection for therapeutic interventions closer to the patients’ home.
Topics out of scope:
- Purely technology driven projects (TRL1-2)
- Development of new technologies (TRL 1-3)
- Explorative projects with no connection to a clinical question
- Interventions or therapies that will be applied within the hospital
- Monitoring of health without an actual measurement (e.g. only questionnaires)
- Infrastructural projects
Additional conditions (in addition to the standard KWF funding conditions):
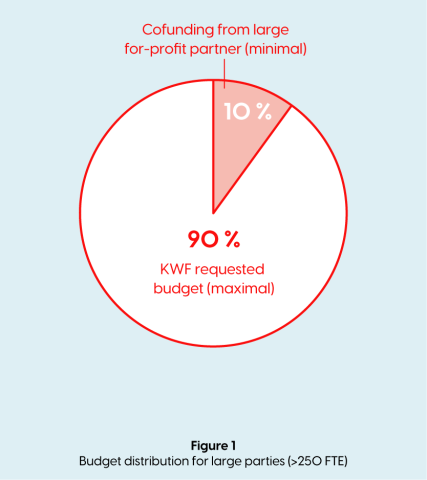
- If the for-profit private partner is >250 FTE, has an active role within the project, this party is considered a participating party and is obligated to make a financial contribution which is ≥10% co-financing of the total project budget (in kind and/or cash). The co-financing is part of the total project budget. (See figure 1)
- If the for-profit private partner is ≤250FTE and >10FTE, has an active role within the project, this party is considered a participating party and the standard KWF funding conditions apply.
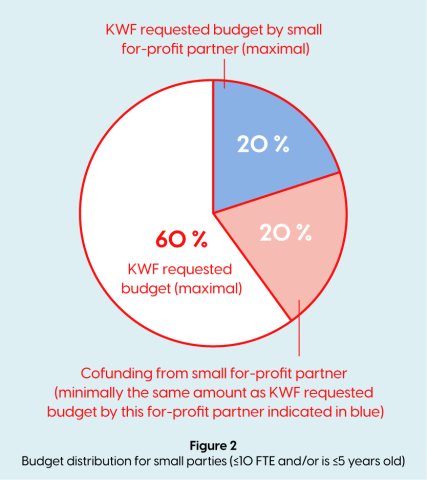
- If a for-profit private partner (SME) is ≤10 FTE and/or is ≤5 years old, has an active role within the project, this party is considered a participating party and can request funding in this call. For-profit private partners can directly request a budget if it directly benefits the research project. SME partners meeting the requirements listed below can apply for funding up to 20% of the total project budget. In addition, an own (in-kind) contribution by the for-profit private partner is required that minimally equals the requested funding by the for-profit private partner. (See figure 2)
Requirements for the SME (≤10 FTE and/or ≤5 years old) for-profit private parties to participate in the project and to be eligible to directly request budget:
- The for-profit private partner is actively participating in the scientifically driven project
- The requested costs are clearly linked to the deliverables and milestones of the project;
- The requested budget by the for-profit private partner needs to be matched by a minimally equal amount (in kind) own contribution (e.g. a small for profit participant of the project requires €300k in materials and FTE to participate in the consortium, 50% of these costs (€150k) can be requested within the KWF budget, as long as €150k =≤ 20% of the total project budget). (See figure 2 above);
- The for-profit private partner is not (yet) profitable;
- The for-profit private partner has ≤10 FTE and/ or is ≤5 years old;
- The maximum requested budget by the for-profit private partner is 20% of the total project budget;
- The for-profit private partner with proprietary technologies from TRL3-TRL 8 are eligible;
- KWF holds the right to request additional information about the for-profit private partner if this is deemed necessary.
Project costs that can directly be requested as a subsidy by participating for-profit private parties include, but are not limited to:
- Materials needed for the project
- Hardware/devices needed in the project.
- Software licenses needed in the project.
- In vivo testing/animal models.
- In vitro testing: outsourcing or purchasing testing kits.
- Toxicity studies.
- Chemical/ physical analysis.
- Data storage/data-infrastructure/servers/databases.
- Scientific and technical personnel/FTE.
- There is a maximum hourly rate for payroll costs:
- Supportive (MBO) € 85,00
- Project Management (HBO) € 100,00
- Specialist (WO) € 125,00
This is the maximum hourly rate excluding VAT and including all other costs (travel costs, parking costs, travel hours, etc.) as they should be applied. If institutes would like to apply a higher hourly rate for motivational reasons, this must always be agreed and approved in advance by KWF.
Materials and services as part of the project costs can be funded based on actual vendor sales prices, not on commercial resale prices of the for-profit private party.
It will always be at the discretion of KWF whether costs incurred by a for-profit private party can be financed by KWF.
Evaluation
KWF uses three review criteria: relevance, scientific quality and feasibility. In addition, the emphasis of this thematic call will be the interaction of an interdisciplinary team and inclusion of patient (organizations) participation. This will be evaluated by interviews in the final assessment phase. A special review committee will be selected for this call, consisting of experts in relevant areas. Additionally, the full proposal will also be reviewed by the patient advocacy committee (PACO).
Timeline
| Call opens: | 5 March 2024 |
| Pre-proposal phase closes: | 7 May 2024 (12.00 noon CET) |
| Call closes: | 10 September 2024 (12.00 noon CET) |
| Consortium interviews: | Mid-November 2024 |
| Funding decision: | December 2024 / January 2025 |
Submitting
Please submit your project proposal via KWF Grant Management System.
Indicative budget and duration:
The indicative total budget is € 5 million. Indicative budget per proposal: €0,2 - 1,5 million, depending on research type (research project or consortium project)
Project duration: 1 - 6 years, depending on research type
More information:
Specific guidelines on the process, characteristics and eligibility terms for this call will be based on the current KWF Guidelines and Funding conditions.
Guidelines will include specific information on application requirements, preferences and recommendations, review procedure and timelines. Please read this document carefully since it contains essential information for timely submission.
Granted KWF projects will be funded under the current Funding Terms and Conditions. Additional project specific conditions may be applied on a case-by-case evaluation.

